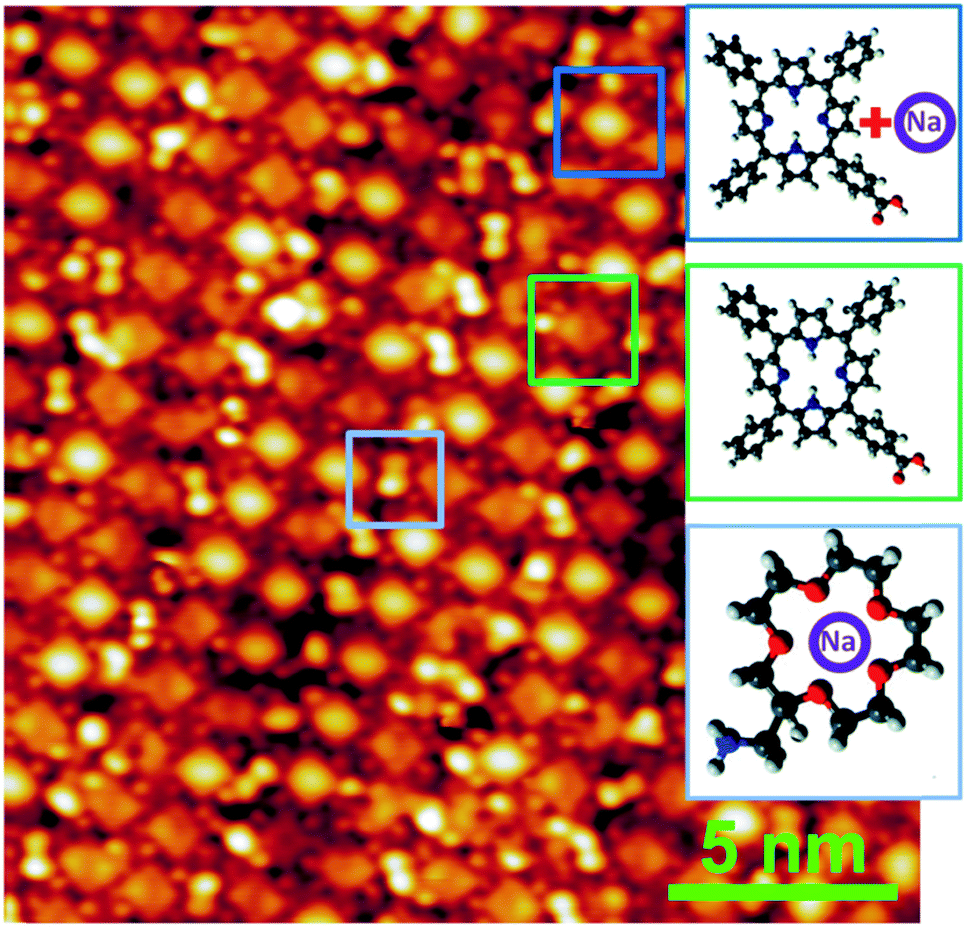
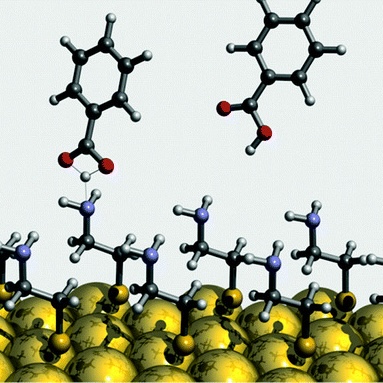
Trapping of alkali atoms by crown ethers
 Our research explores host-guest chemistry and molecular recognition at surfaces, focusing on how tailored molecular
architectures can control interactions at the nanoscale. By designing and assembling supramolecular networks on metallic
and insulating surfaces, we investigate the mechanisms driving molecular confinement, selective adsorption, and dynamic
host-guest interactions.
Our research explores host-guest chemistry and molecular recognition at surfaces, focusing on how tailored molecular
architectures can control interactions at the nanoscale. By designing and assembling supramolecular networks on metallic
and insulating surfaces, we investigate the mechanisms driving molecular confinement, selective adsorption, and dynamic
host-guest interactions.
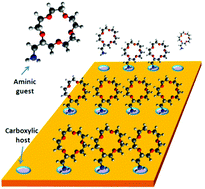
In our studies, we have examined porous molecular frameworks capable of trapping guest molecules, enabling selective
recognition through non-covalent interactions such as hydrogen bonding, metal coordination, and van der Waals forces.
These systems, often based on organic linkers and functionalized macrocycles, serve as platforms for studying adsorption
phenomena and templated self-assembly. For instance, we have explored molecular traps for fullerenes and
coordination-driven frameworks that exhibit adaptive host-guest behavior.
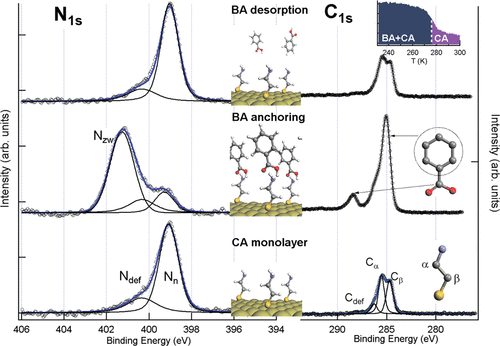
To characterize these interactions, we utilize spectroscopic and microscopic techniques to probe molecular organization
and stability at surfaces. Our combined experimental and theoretical approach provides insights into the key factors
governing chemical recognition, with potential applications in molecular sensing, catalysis, and nanostructured material
design. By advancing the understanding of host-guest chemistry at interfaces, we contribute to the development of
functional molecular architectures for future nanotechnology applications.