Navigazione principale
Navigazione principale
PhD Top Stories
Maria Michelina Raso
GMMA and Glycoconjugate Approaches Compared in Mice for the Development of a Vaccine against Shigella flexneri Serotype 6
Doctoral Programme in Molecular Biomedicine
Shigella infections are one of the top causes of moderate to severe diarrhea (MSD) throughout the world. The Global Burden of Disease Study 2016 estimates approximately 270 million cases with 212,000 total deaths per year, of which 30% are children younger than 5 years especially in low- and middle-income countries (LMIC). There are four different Shigella species and more than 50 different serotypes; among them S. sonnei and S. flexneri are predominant but their prevalence changes in different countries, over time and with the economic development. Currently, no vaccines are widely available and increasing levels of antimicrobial-resistance make Shigella a high priority for vaccine development. The serotype-specific O-antigen (OAg) moiety of Shigella lipopolysaccharide has been recognized as a key target for protective immunity, and many OAg-based candidate vaccines are in development. S. flexneri 6 OAg, one of the main serotype prevalent in LMIC, is characterized by a linear polysaccharide backbone [?2)-a-L-RhapIII-(1?2)-a-L-RhapII-(1?4)-▀-D-GalpA-(1?3)-▀-D-GalpNAc-(1?], with RhaIII variably O-acetylated in position 3 or 4. The OAg biosynthesis depends on the Wzx/Wzy pathway and involves the first step of polymerization when the OAg chain length is regulated by the Wzz proteins, responsible for unique polysaccharide modal lengths. The OAg repeating units can also be polymerized into a group 4 capsule (G4C) because of the presence of a G4C operon, in addition to the wzx-wzy cluster (Figure1).
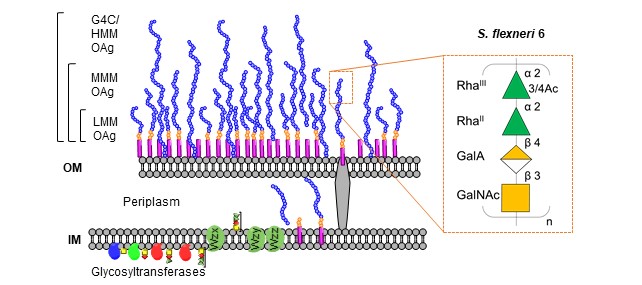
Figure 1: OAg moieties expressed on the outer membrane (OM) of S. flexneri 6 bacteria, displaying G4C/ high molecular mass (HMM) OAg, medium molecular mass (MMM) OAg and low molecular mass (LMM) OAg.
A well-established technology for the development of OAg-based vaccines is the glycoconjugation approach. The covalent linkage of a polysaccharide (PS) to an appropriate carrier protein converts the PS from a T-independent to a T-dependent antigen, able to induce immunological memory, thus rendering the vaccine effective also in infants. Recently, the Generalized Modules for Membrane Antigens (GMMA) technology has been proposed as an alternative approach to traditional glycoconjugate vaccines for OAg delivery. GMMA are outer membrane exosomes naturally released from genetically engineered Gram-negative bacteria, where the OAg is displayed in its natural outer membrane context. Bacteria are mutated in order to increase exosome formation, through the deletion of the tolR gene, and to reduce potential reactogenicity, usually through reduction of acyl chains in the lipid A structure by deletion of the htrB, msbB or pagP genes.
These two technologies were compared for a vaccine against S. flexneri serotype 6. Genetic strategies for GMMA production, conjugation approaches for linkage of the OAg to CRM197 carrier protein, and a large panel of analytical methods for full vaccine candidates characterization have been put in place. In a head-to-head immunogenicity study in mice, GMMA induced higher anti-OAg IgG than glycoconjugate administered without adjuvant. This was confirmed also by the ability of the sera to kill bacteria using the Serum Bactericidal Activity (SBA) analysis. When formulated on adjuvant, glyconjugate induced higher anti-OAg IgG than GMMA, while SBA titers remained similar (Figure 2). Both GMMA and the glycoconjugate were able to induce persistent responses in mice up to 98 days post first injection.
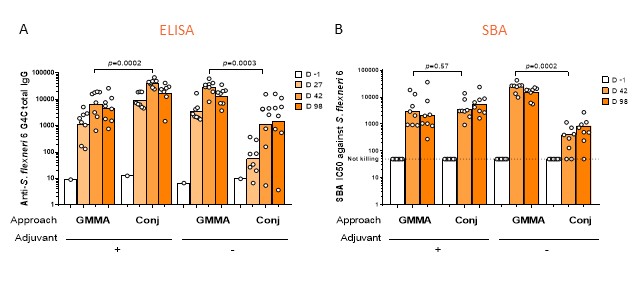
Figure 2: S. flexneri 6 glycoconjugate (Conj) and GMMA displaying MMM OAg compared in mice. Eight mice per group were subcutaneously immunized at days 0 and 28, with 1 Ág OAg dose with or without Alum. Anti-OAg specific IgG geometric mean units (bars) and individual antibody levels (dots) (A); SBA titers of single sera against S. flexneri serotype 6 strain (B).
Glycoconjugates are a well-established bacterial vaccine approach, but can be costly, particularly when multicomponent preparations are required. With similar immunogenicity and a simpler manufacturing proces, GMMA are a promising strategy for the development of a vaccine against Shigella.
Authors and affiliations
Maria Michelina Raso1,2, Gianmarco Gasperini1, Renzo Alfini1, Fabiola Schiavo1, Maria Grazia Aruta1, Martina Carducci1, Maria Concetta Forgione3, Silvia Martini3, Paola Cescutti2, Francesca Necchi1, Francesca Micoli1,2Department of Life Science, University of Trieste, Building C11, via L. Giorgieri 1, 34127-Trieste, Italy
3GSK, via Fiorentina 1, 53100-Siena, Italy
Contact
Maria Michelina Raso, email: mariamichelina.raso@phd.units.itReference
Vaccines 8, 160 (2020)
DOI: 10.3390/vaccines8020160
Informazioni aggiornate al: 12.11.2021 alle ore 13:54
Contact: Webmaster - Università di Trieste pagina curata da: Research Doctorate



