Navigazione principale
Navigazione principale
PhD Top Stories
Rebecca Bertolio
SREBP1 COUPLES MECHANICAL CUES TO LIPID METABOLISM
Doctoral Programme in Molecular biomedicine
A balanced tissue rigidity is a key feature of organs proper function. However, it has been observed that the normal tissue stiffness is often compromised in several pathologies. For example, solid tumors are stiffer than normal tissues, thus allowing their aberrant growth and their malignant phenotype, suggesting a connection between tissue stiffness and tumorigenesis. Similarly, also other pathologies, such as fibrosis, are characterized by a damaged tissue, which display a higher stiffness grade compared to the normal counterpart. The excessive and continuous mechanical stimulation of cells exerted by tissue rigidity, has been demonstrated to compromise organs function, as occurs in late stages of pathologies of the liver, kidneys, lungs and heart. Molecular mechanism responsible for enhancing tissue rigidity are not well clear so far, but is progressively more evident the importance of cell metabolism in response to mechanical cues. Thus, understanding which molecules and key processes are involved in the response to mechanical cues might be relevant to develop therapeutic strategies for pathologies such as fibrosis and cancer.
In this study, SREBP1, a well-known protein involved in lipid metabolism regulation, has been identified as a link between mechanical cues and lipid biosynthesis. It has been demonstrated that mechanical regulation, exerted by high tissue stiffness, and mechanotransduction, via actin cytoskeleton, are able to turn off lipid biosynthesis, either in human mammary gland-, liver- and lung-derived cells, and in animal models such as Drosophila melanogaster (Figure 1).
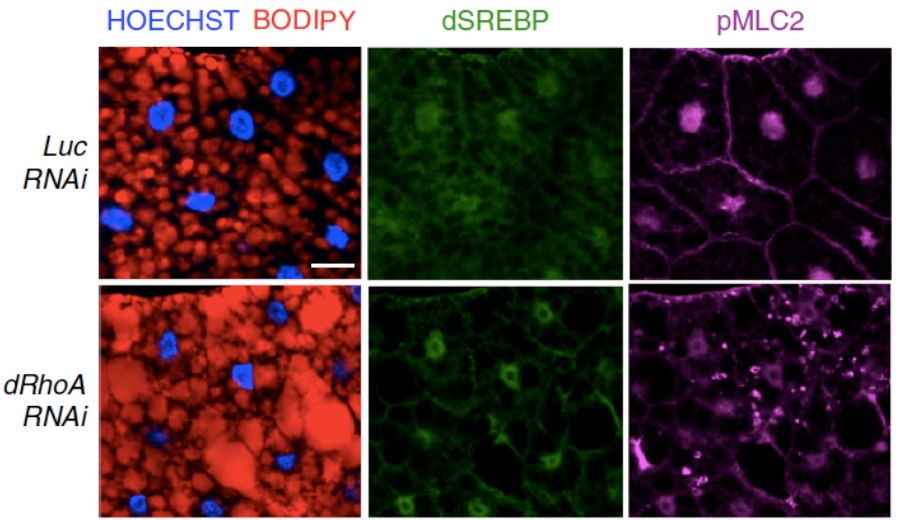
Figure 1: Immunofluorescence of Drosophila larval fat bodies in normal (Luc RNAi) and altered mechanotransduction (dRhoA RNAi) – in red: lipid droplets (BODIPY); in green: SREBP1; in magenta: pMLC2 (marker of actin cytoskeleton); in blue: nuclei (HOECHST). Scale bar, 20 µm.
Acto-myosin dynamics drain an important fraction of cellular energy, yet it is unknown how the cell derives the vast amount of energy it needs to support these processes. It has been demonstrated that activation of AMPK is a key player in a junctional contractility pathway that increases glucose uptake and energy production, in terms of ATP synthesis. The finding that SREBP1-dependent lipid anabolism is prevented by cytoskeleton contraction via activation of AMPK suggests that this mechanism could be important to integrate environmental mechanical signals with intracellular requirements, allowing a cell to maintain an optimal energy status.
As shown by this work, the control of SREBP1 by acto-myosin dynamics may represent a fundamental mechanism to regulate cell behavior. Indeed, it has been demonstrated that mechanical cues are instrumental for stem cell to differentiate into osteocytes (stiff environment) or adipocytes (soft environment). Furthermore, SREBP1 has been shown to promote adipocyte differentiation. To support this notion, I found out that it is in response to altered mechanical cues that SREBP1 activation determines the fate into adipocytes of murine mesenchymal stem cells (mMSCs) (Figure 2).
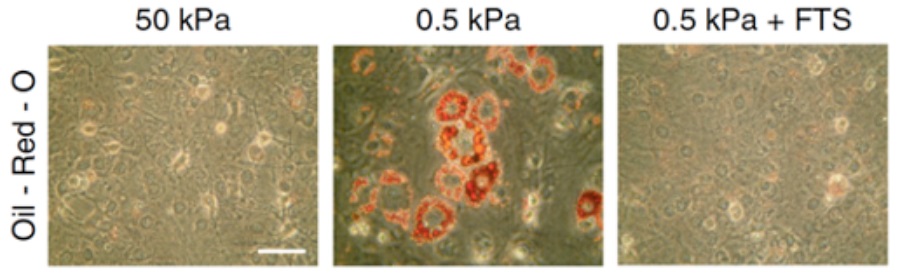
Figure 2: Oil-Red-O staining of lipid droplets (in red) in mMSCs cultured on either stiff (50 kPa elastic modulus) or soft (0.5 kPa elastic modulus) fibronectin-coated hydrogel matrix, or soft (0.5 kPa elastic modulus) fibronectin-coated hydrogel matrix with fatostatin treatment, a SREBP inhibitor (FTS). Scale bar, 100 µm
As said above, I’ve found that acto-myosin contractility regulates Drosophila fat storage via SREBP-dependent lipid biosynthesis and accumulation, supporting that coordination between mechanical and metabolic pathways may have implications at the organismal level, such as in human physio-pathologic conditions. Indeed, I’ve found that in breast and lung tissues with altered ECM deposition and stiffening of the surrounding environment, in terms of mammographic density and Idiopathic Pulmonary Fibrosis, SREBP1 signature is downregulated, supporting the idea that an increased mechanotransduction is altering somehow the correct physiological regulation of metabolic processes. This mechano-dependent regulation of lipid metabolism might resemble a new therapeutic approach in such diseases, by restoring the correct properties of the ECM and thus the proper metabolic homeostasis of the cell.
Authors and affiliations
Rebecca Bertolio1/2, Francesco Napoletano1/2, Miguel Mano3, Sebastian Maurer-Stroh4/5, Marco Fantuz1/6, Alessandro Zannini1/2, Silvio Bicciato7, Giovanni Sorrentino1#, Giannino Del Sal1,2,82Dipartimento di Scienze della Vita, Universitŕ degli Studi di Trieste, Trieste, Italy.
3Center for Neuroscience and Cell Biology, University of Coimbra, Coimbra, Portugal.
4Bioinformatics Institute (BII), Agency for Science Technology and Research (A*STAR), 30 Biopolis Street, #07-01 Matrix, 138671, Singapore.
5Department of Biological Sciences (DBS), National University of Singapore (NUS), 14 Science Drive 4, 117543, Singapore.
6International School for Advanced Studies (SISSA), Trieste, Italy.
7Department of Life Sciences, University of Modena and Reggio Emilia, Modena, Italy.
8IFOM, the FIRC Institute of Molecular Oncology, Via Adamello, 16-20139, Milan, Italy.
#Current address: Laboratory of Metabolic Signaling, Institute of Bioengineering, Ecole Polytechnique Fédérale de Lausanne, CH-1015 Lausanne, Switzerland.
Contact
Rebecca Bertolio, email: rbertolio@units.itReference
Bertolio Rebecca, Napoletano Francesco, Mano Miguel, Maurer-Stroh Sebastian, Zannini Alessandro, Fantuz Marco, Bicciato Silvio, Sorrentino Giovanni and Del Sal Giannino.Sterol regulatory element binding protein 1 couples mechanical cues to lipid metabolism
Nature Communications 10, 1326 (2020)
DOI: 10.1038/s41467-019-09152-7
Informazioni aggiornate al: 19.8.2020 alle ore 09:18
Contact: Webmaster - Università di Trieste pagina curata da: Research Doctorate



